- 1 1. Introduction
- 2 2. Treatment objectives and characteristics of the wastewater
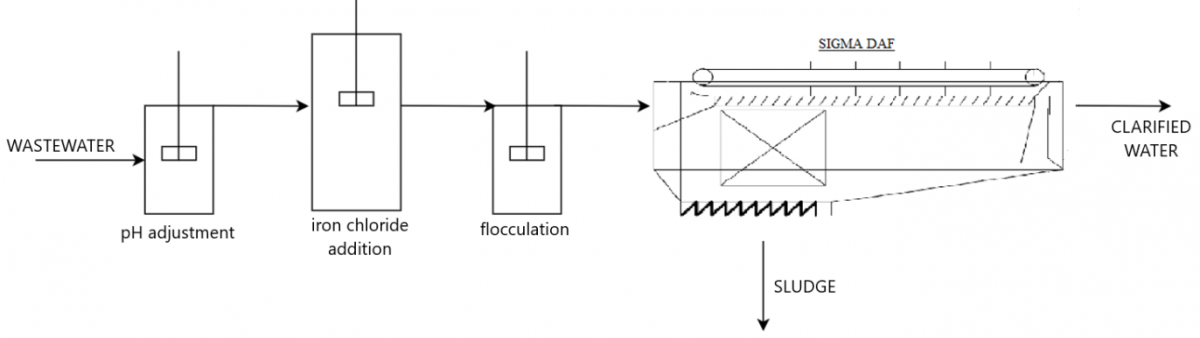
- 3 3. Proposed technology
- 4 4. Dosages and retention times estimation
- 5 5. Process control: flowrates, iron dosage, pH adjustment and flocculation
- 6 6. Follow-up instruction and selenium analysis
- 7 7. Treatment alternatives: applying formulated products as coagulants and flocculants
- 8 8. References
14 April 2021
Selenium removal from industrial wastewater with iron co-precipitation
Category:
Engineering for the removal of high concentrations of selenium in wastewater with iron co-precipitation: Application of SIGMA DAF systems and SMARTDAF technology.
1. Introduction
This document is a technological study for the removal of high and fluctuating selenium concentrations in industrial wastewater.
Elemental selenium is an essential nutrient in living beings in very low concentrations, ranging micrograms per litre, but in high concentrations it presents a serious risk of damage in health and the environment.
Some industrial processes can result in selenium concentrations in their wastewater above permissible levels, therefore the discharge of selenium to the environment is regulated by the competent environmental authorities of each country.


The current available technologies and that have proven highly effective in removing heavy metals in general and selenium in particular from industrial wastewater are the following:
- Chemical precipitation and co-precipitation.
- Adsorption in activated carbon.
- Ion exchange.
- Reverse osmosis.
- Biological treatment (MBBR, ABMet, etc).
- Other processes of high complexity and cost.
Among these technologies, the most commonly applied for heavy metal removal in general is chemical precipitation and co-precipitation.
For the specific removal of selenium, the most commonly used precipitant is iron hydroxide Fe(OH)3 generated by adding iron salts to the aqueous medium, generally in the form of iron chloride FeCl3.
SIGMA has studied this solution in depth and proposes selenium removal by co-precipitation with iron, through a studied engineering of physicochemical treatment of wastewater.
2. Treatment objectives and characteristics of the wastewater
Many industries, given the characteristics of their processes, discharge wastewater with a very high concentration of heavy metals. Sometimes the predominant metal is selenium.
For example, wastewater from the mining industry is known to contain very high concentrations of selenium, generally ranging from 0.03 to 2 mg Se / L.
An alarmingly high concentration can be considered when the wastewater has an average concentration of >2 mg / L selenium.
Surprisingly, SIGMA has found industrial wastewater whose selenium content reaches up to 80 mg / L, the content being highly fluctuating and difficult to predict. These cases require a continuous adjustment of the reagent dosage for the elimination of the selenium present in the water. This article focuses largely on tackling this problem that some industrial sectors have.
These concentrations are taken as the calculation basis in this article to describe the engineering proposed by SIGMA.
The predominant form of selenium is expected to be in the form of selenite (selenium IV).
SIGMA's engineering performance objective is the removal of at least 80% of the selenium present in wastewater.
3. Proposed technology
SIGMA applies its experience and technological knowledge to the process design for the removal of selenium with iron co-precipitation, dosing iron chloride FeCl3 as precipitating and coagulating agent.
The technologies applied by SIGMA for this process are:
- Physical-chemical system for iron co-precipitation, coagulation and flocculation of selenium and other pollutants in the wastewater (COD, BOD5, TSS, fats and greases)
- Solids and flocs separation and clarification in a dissolved air flotation SIGMA system.
- The whole process is intensively followed-up and controlled with the SMARTDAF technology.
The iron precipitation reaction sequence in aqueous medium is as follows, starting with the addition of iron chloride to the wastewater:
FeCl3 → Fe3+ + 3Cl-
Fe3+ + 3H2O → Fe(OH)3(s) + 3 H+
The precipitation of iron hydroxide Fe (OH)3 carries heavy metals and other solids by co-precipitation. It is especially effective for selenium (both particulate and dissolved in the form of selenite IV) and is performed by the effect of adsorption of the metal to the Fe (OH)3 precipitate. Other major constituents of the water matrix generally do not interfere with adsorption.
The pH decreases automatically with the addition of iron salts as observed in the reaction. It is necessary to maintain the pH in the appropriate range to avoid destabilization of Fe (OH)3 and consequently the re-dissolution of selenium in the water, this has to be maintained throughout the whole process.
The pH range that has been recorded in historical data and experience presents optimal pH values between 5.0 and 5.5, although in some cases a broader range between 4.0 and 6.0 is contemplated. In any case, the pH should not drop below 4.0 or rise above 6.0 in order to not reduce the effectiveness of the treatment.
To maintain the pH in this range, soda (NaOH solution) will be added as a support to increase the pH in case the FeCl3 dosage implies a drop in the pH below the optimal range.
The pH control and FeCl3 and soda dosage must be fed back. Automation of the addition of FeCl3 and soda is recommended by controlling pH = 5.0 - 5.5 and initial and final concentration of selenium. It is recommended to control other pollutant values such as turbidity, total suspended solids and other pollutants of interest. SIGMA offers this control system as SMARTDAF control and it is explained below.
After the pH control and adjustment and the addition of FeCl3 (which acts as a general coagulant also for suspended solids, oils and fats and other contaminants present in the wastewater), a flocculant (polyelectrolyte) will be added to increase the efficiency of floc formation, that will be eliminated in the DAF system.
In case it is necessary to re-adjust the pH to the requirements of the plant, this will be done in the clarified water at the DAF outlet once the solids have been removed. The pH adjustment to the required values for the discharge must not to be done earlier in the process, since it could lead to destabilization of the precipitates generated and re-dissolution of the selenium in the water.
4. Dosages and retention times estimation
Sometimes it is difficult to establish an optimal dosage for wastewater whose selenium content is highly fluctuating. SIGMA calculates an estimation of the dosage of iron chloride, based on historical data and experience by a correlation of iron concentration - selenium elimination, and from these empirical and experienced data, the treatment and control systems are designed.
The FeCl3 dosage estimation has been carried out by treating data collected from laboratory studies, pilot plant studies, plants operating on an industrial scale, and historical data and SIGMA experience. All these data sources have demonstrated a very adequate selenium removal performance in industrial wastewater by applying iron co-precipitation.
The most representative data are collected in Table 1 as the necessary ratio of iron [initial total Fe3+ / Se] in concentration for the elimination of between 60% and 95% of initial total selenium. Selenium values range from trace concentrations to very high concentrations in order to visualize the full spectrum and the relationship to iron dosage.
Table 1. Fe3+ / Se ratio applied for different values of initial total selenium removal in industrial wastewater by co-precipitation.

With these values, the Fe3+ requirement curves are represented and the values that result in a selenium removal of between 85% and 95% are extrapolated as shown in Figure 2.
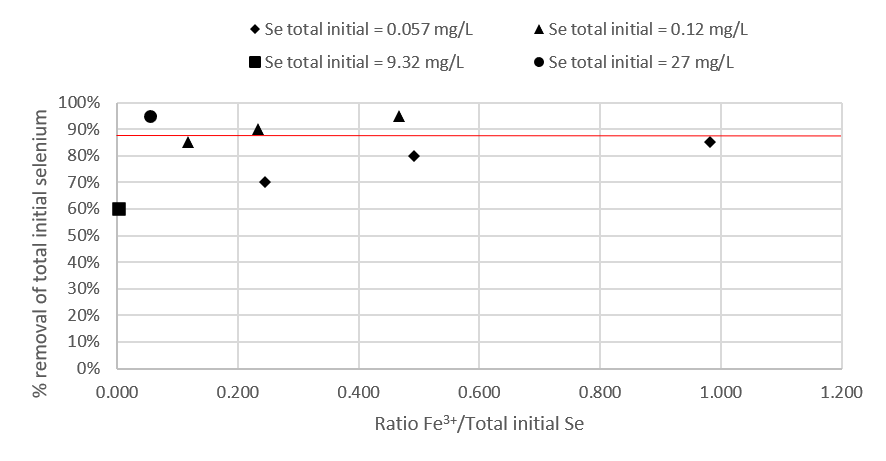
The values corresponding to the extrapolation line of Figure 2 are selected from Table 1 and are represented as a function of the initial total selenium concentration in the water to be treated. The regression obtained, represented in Figure 3, allows the estimation of Fe3+ necessary for the average concentration of selenium in the wastewater. The equivalent concentration in the pure FeCl3 iron chloride product is also calculated.
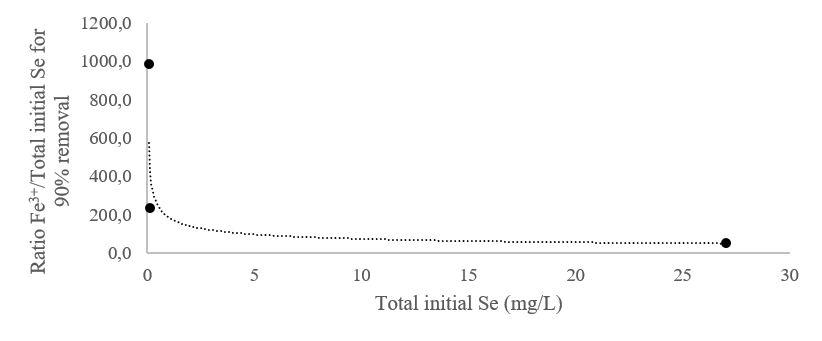
For example, a concentration of 7600 mg/L of FeCl3 required for removal of 85% - 95% of initial total selenium of 80 mg / L is calculated.
The required reaction time is estimated to be 80 minutes as a starting point.
5. Process control: flowrates, iron dosage, pH adjustment and flocculation
The efficiency of the removal of selenium with iron co-precipitation depends on the oxidation state of the selenium, the initial selenium concentration, the continuous adjustment of the Fe3+ dosage depending on the fluctuations of the discharge characteristics, pH and composition of the matrix.
The study presented in this article is based on proven data and although it can be used as a starting point in the design of the technology, it will be absolutely necessary to continuously readjust the chemical dosage and retention times, which must be carried out simultaneously with the operation of the proposed system, using the SMARTDAF control system and methodology specially designed and offered by SIGMA.
The DAF coagulation-flocculation and pH control and clarification system is made up of flexible equipment and instrumentation, which can be adapted to operate for 8, 16 or 24 hours a day.
The wastewater is collected in one or more accumulation tanks, depending on the flow to be treated. These tanks have agitation equipment that guarantees a correct mixing of the water in anoxic conditions without incurring in strictly anaerobic conditions. Once the water has been collected in these tanks, a measurement of the selenium content will be carried out, detailed in section 6 of this article.
Each tank has pH measurement equipment connected to soda dosing systems to adjust its value so that a stable medium is available in the following reactors with a value of approximately 5.5, never exceeding values of 6.0.
From here, the physical-chemical treatment will be carried out with the dosage of iron chloride, with special importance being the contact time between this reagent and the water to be treated in the coagulation reactor, followed by flocculation and final clarification in a SIGMA DAF system.
Calculating the volume of the coagulation reactor is key to effective selenium reduction. The volume of the tank is overestimated depending on the flow to be treated, the time established for the treatment and the required contact time.
The calculations are made from a treatment guarantee point of view, i.e. the volumes of the rectors are overestimated to allow a margin of safety regarding the discharge flow rate and to allow enough time for the laboratory analysis of the selenium control. as will be described below.
The entire installation offered by SIGMA has the necessary measurement equipment that allows its total automation and management, including a programmed PLC.
The SMARTDAF integrated management system is governed by the following control cycle:
- Control and adjustment of the effluent from the accumulation tanks entering the coagulation-flocculation system.
- pH measurement and soda dosing adjustment to keep its value around 5.5.
- Iron chloride dosing and control applying a calibration curve (% selenium removal – Fe3+ dosing) as shown in Figure 4 for the desired removal performance. This must be complemented by the laboratory analysis of selenium, described below in paragraph 6.
Repeated execution of this relationship and analysis will lead to the generation of a curve that is more and more adapted to the process and wastewater, governed by the following function:
% selenium removal = f (Fe3+ dosage)
This function shall be more and more exact as less fluctuant in the initial selenium concentration in the wastewater.
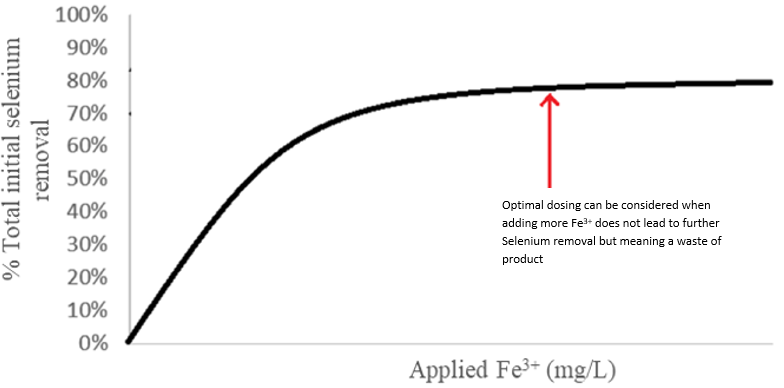
Another function that must be applied is the control in the fluctuation of the pH when adding iron chloride, which must be complemented with the registered values of soda dosage and pH value of the previous point.
In this way, the pH and selenium removal control is intrinsically linked. An automated adjustment based on these dose-response curves is established by the SMARTDAF system for the following conditions:
- pH value preferably of 5.5, without dropping below 4.0 or rising above 6.0, giving an optimal elimination of selenium. An optimal dosage will be considered when, despite adding more concentration of Fe3+, the removal of selenium is similar or not significantly higher (a flat zone is reached in the ratio % removal of selenium - dosage of Fe3+).
In addition, the SMARTDAF system allows the control and adjustment of the rest of the process:
- Measurement and control of the dose of flocculant as polyelectrolyte: this step follows a similar reasoning than the ratio of Fe3+ and selenium elimination, but in this case it is applied to the effective elimination of total suspended solids and the correct functioning of the DAF clarification system.
- Control and adjustment of the DAF saturation and aeration system.
Once the curves are sufficiently adequate, the dosages will be self-adjusted from the values given by the operator of the concentration of total selenium in raw water and of total suspended solids.
When the SMARTDAF integrated management system shows continuous results, the control of variables will be expanded to turbidity, nitrogen content (total, nitrates and ammonium) and phosphorus content (total and phosphates) as other indicators of process efficiency.
The sludge generated in this treatment is stored in a sludge tank and treated using the most appropriate technologies for the type of sludge offered by SIGMA:
- Filter press: can reach a dry matter concentration of 35%, requires dosing of lime.
- Decanter centrifuge: it can reach a dry matter concentration of 15%. It is more comfortable, clean and automatable.
6. Follow-up instruction and selenium analysis
In order to implement the chemical dosage control and readjustment described in section 5, a scheduled control of selenium is absolutely necessary.
Selenium is an element that cannot be effectively measured in continuous-like basis, it must be analysed manually using a special method in the laboratory. This analysis consists sampling, digestion and treatment of this sample, the measurement of the selenium concentration applying a spectrophotometric method and the treatment of the data obtained.
The duration of the analysis from taking the sample to obtaining a numerical value of concentration will depend on the skills of the person doing the work, estimated at three hours, maximum.
SIGMA designs a hydraulic configuration in the accumulation tanks so that there is a sufficient time margin to measure the initial selenium concentration and to reconfigure the chemical dosages to achieve at least 80% selenium removal.
In addition, SIGMA offers its SIGMA LAB laboratory facilities for the analysis of selenium concentration.
The steps to follow are the following:
Sampling
Sample collection of the initial selenium analysis in the wastewater and sample collection of the treatment effluent in order to calculate the removal performance and feed the curve % removal of selenium - Fe3+ dosage.
The wastewater sample must be taken from the raw water before entering the treatment system so that it is as little disturbed as possible, and the sample for the elimination control will be taken from the clarified water at the exit of the DAF system.
The following steps will be carried out in a similar way for each sample, either from the wastewater or from the clarified one.
Samples dilution
The spectrophotometric method of analysis of selenium works in a range of with a stipulated maximum detectable selenium, generally of 2 ppm, therefore, the waste water sample must be diluted according to the estimated initial selenium concentration in the raw water.
For example, for an expected selenium concentration of 80 ppm, the sample will need to be diluted at least 40 times. It is recommended to keep (or run in parallel) dilutions above and below factor 40 to ensure that at least one of the aliquots has the appropriate concentration for the test method. For each dilution, it is recommended to have at least three aliquots to have enough data to average. The experience in the generation of raw water and the handling of the analysis method will allow in the future to be able to predict which dilution will be the correct one. Also, experience will make it possible to predict the appropriate dilution for the sample from the DAF outlet.
Samples digestion
The already diluted aliquots will be introduced to a treatment and digestion applying reagents and materials described in detail in the manual of the spectrophotometric method of analysis of selenium offered by SIGMA.
Concentration value
Once the spectrophotometry equipment returns a selenium concentration value, this value must be multiplied by the times that the corresponding aliquot was diluted. Only valid data will be considered, i.e., those corresponding to aliquots that are within the detection range of the spectrophotometric equipment.
Data treatment
For each aliquot that has generated valid data, an average initial selenium concentration must be calculated. This value will be entered in the SMARTDAF re-adjustment system to dose the chemicals according to the concentration of selenium to be treated.
Yield can be calculated using the following relationship:
SeT0: total selenium concentration in the wastewater, in ppm.
Seclar: total selenium concentration in the clarified water effluent from the DAF, in ppm.
Once the system has been re-adjusted initially, it will follow the pattern described in section 5 for the optimization of the operation.
Each of these values will be accumulated in a database to, as the wastewater treatments are carried out, to obtain an increasingly precise curve of chemical dosage (re-adjusted by SMARTDAF) with respect to the concentration of initial total selenium, specially adjusted for each specific case of wastewater.
7. Treatment alternatives: applying formulated products as coagulants and flocculants
The chemical dosage proposal has been made based on the philosophy of applying generic products, in this case an iron salt that can be found as iron chloride as commercial product. Its price is dependent on the supplier, but always is significantly lower than the price of the formulated products.
A formulated product is a product with a specific trademark whose active principle (Fe3+ in this case) is embedded in a unique matrix. The constituents of this matrix are not accessible knowledge since it is the particularity by which the product has commercial value and is part of the confidential information of the manufacturer and / or supplier.
The formulated products have the advantage, with respect to the generic ones, that their performance is remarkably higher, which is why a lower consumption is needed.
Given the nature of these products, the generated sludge must be treated as special waste, its subsequent treatment being mandatory.
It is very important to keep in mind that both the know-how of coagulation - flocculation processes and the manufacturers of all the products described, recommend applying JAR-TESTS analysis prior to adjusting the dosage of the set of products to be applied.
SIGMA offers JAR-TEST services at its SIGMA LAB facilities.
8. References
Bhattacharyya D., Jumawan A.B., Grieves R.B. (1979) Separation of Toxic Heavy Metals by Sulfide Precipitation. Separation Science and Technology. 14(5), 441-452.
McCloskey C. (2009) Selenium Removal from Refinery Wastewater via Iron Co-Precipitation in a Mobile Clarifier. Water Environmental Federation. Microconstituents/Industrial Water Quality 2009. 439-445.
Merrill D., Manzione M., Parker D., Petersen J., Chow W., Hobbs A. (1987) Field Evaluation of Arsenic and Selenium Removal by Iron Coprecipitation. Reprinted from Journal of the Water Pollution Control Federation. Environmental Progress. 6(2), 82-90.
Metcalf & Eddy (2014) Wastewater Engineering: Treatment and Resource Recovery. Fifth Edition. MCGraw-Hill Education.
Overman S.D. (1999). Process for removing selenium from refinery process water and waste water streams. Canadian Intelectual Property Office. International Patent Classification: C02F 1/72, 1/52. International publication number: WO 99/20569.
Sandy T. (2010). Review of Available Technologies for the Removal of Selenium from Water. CH2M HILL. North American Metals Council.